Have you ever wondered, “Is diamond a compound?” You’re not alone!
Many people are curious about what makes this precious gemstone so special.
In this guide, we’ll explore the true nature of diamonds.
Let’s dive in and uncover the science behind their sparkle!
What Is a Diamond?
Diamonds are one of the world’s most precious gemstones. But scientifically, what are they?
- Pure Carbon: Diamonds are made entirely of carbon atoms.
- Unique Structure: These carbon atoms are arranged in a specific way, giving diamonds their exceptional properties.
💎 Curious about the value of diamonds? Check out why diamonds are expensive.
Is Diamond a Compound or an Element?
Diamond Is an Allotrope of Carbon
- Not a Compound: Diamonds are not a compound; they are a form of pure carbon.
- Allotrope Defined: Allotropes are different forms of the same element, where atoms are arranged differently.
Other examples of carbon allotropes include:
- Graphite: Found in pencils, with carbon atoms arranged in layers.
- Fullerenes: Carbon atoms arranged in a spherical shape.
💡 Learn about different types of jewelry materials.
Understanding Elements and Compounds
What Is an Element?
- Pure Substance: Made of only one type of atom.
- Cannot Be Broken Down: Elements cannot be separated into simpler substances.
- Examples: Carbon (C), Oxygen (O), Gold (Au).
What Is a Compound?
- Combination of Elements: Made of two or more different elements chemically bonded together.
- Fixed Ratios: Compounds have elements in specific proportions.
- Examples: Water (H₂O), Carbon Dioxide (CO₂).
Why Isn’t Diamond a Compound?
- Single Element: Diamonds consist only of carbon atoms.
- No Other Elements: There’s no chemical bonding with other elements.
💎 Conclusion: Diamonds are a pure element, not a compound.
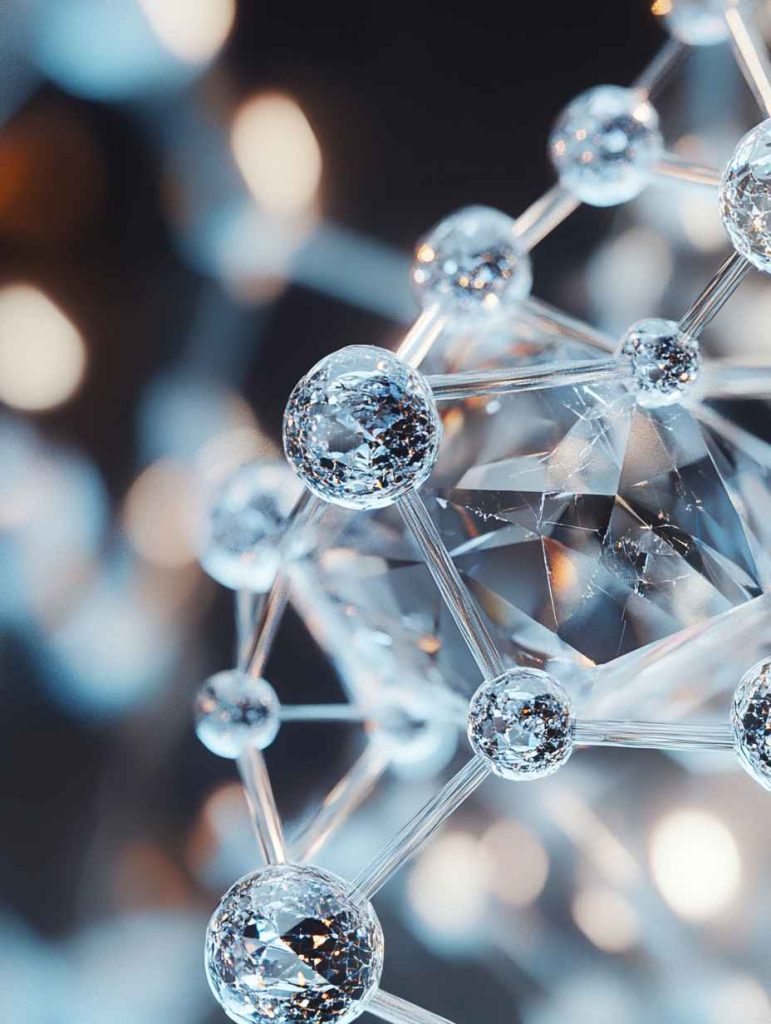
The Structure of Diamond
How Are Carbon Atoms Arranged?
- Tetrahedral Lattice: Each carbon atom is bonded to four others.
- Strong Bonds: This structure makes diamonds incredibly hard.
- Three-Dimensional Network: Creates the rigid, repeating pattern unique to diamonds.
Properties Resulting from the Structure
- Hardness: The hardest natural material.
- High Melting Point: Resistant to heat due to strong bonds.
- Transparency: Allows light to pass through, creating that iconic sparkle.
💡 Want to know how diamonds compare to other gems? Read about gemstones.
Diamond vs. Graphite
Both are made of carbon, but they couldn’t be more different!
| Property | Diamond | Graphite |
|---|---|---|
| Structure | 3D lattice | Layers of carbon atoms |
| Hardness | Extremely hard | Soft, easily breakable |
| Transparency | Transparent and sparkly | Opaque |
Common Questions About Diamonds
Q: Can Diamonds Conduct Electricity?
A: No, diamonds don’t conduct electricity because they have no free electrons.
Q: Why Are Diamonds So Valuable?
A: Their rarity, beauty, and unique properties make them precious.
Q: Are Lab-Grown Diamonds Real Diamonds?
A: Yes, they have the same chemical composition and properties as natural diamonds.
💎 Learn more about diamond authenticity tests.
Interesting Facts About Diamonds
- Billions of Years Old: Most diamonds were formed deep within the Earth’s mantle billions of years ago.
- 100% Carbon: Made purely of carbon atoms, nothing else.
- Beyond Jewelry: Diamonds are used in cutting tools because of their incredible hardness.
Final Thoughts
So, is diamond a compound? The answer is no. Diamonds are a pure element, an allotrope of carbon with a unique structure that makes them extraordinary.
Key Takeaways
- Diamond Is an Element: Made entirely of carbon.
- Not a Compound: No other elements are combined with carbon.
- Special Properties: Hardness, transparency, and brilliance make diamonds stand out.
💡 Ready to explore more? Check out why gold and diamonds are valuable.
Got questions? Share them below—we’d love to help! ✨

